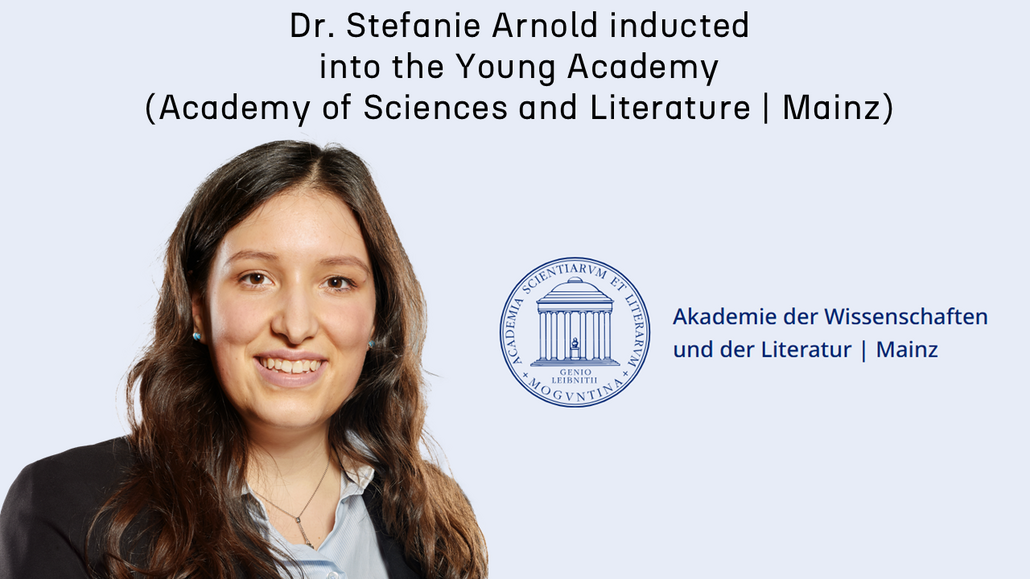
Congratulations to Stefanie to be admitted to the Young Academy (Academy of Sciences and Literature | Mainz)! Since 2016, The Young Academy | Mainz has been the Academy’s dedicated programme for supporting emerging talent. Membership gives selected scientists, writers, and musicians the chance to build an interdisciplinary network and engage across fields. A key principle is the full and equal involvement of Young Academy members in the Academy’s activities. Active participation in meetings and events – and regular exchange with members of the established scholarly society – are therefore central to the programme. Members are also encouraged to shape the agenda themselves by organising events and launching new initiatives, especially through interdisciplinary working groups that pursue timely research questions developed independently by the members.